The Health Data Research Service: a foundation for innovation

In this blog, Dr Emma Lawrence, Head of data tech policy and public affairs at BIA, writes about the UK government's recent £600 million initiative to launch a Health Data Research Service which aims to revolutionize access to NHS data and tackle longstanding governance and technical hurdles.
The Government has announced funding to set up a Health Data Research Service (HDRS), in collaboration with the Wellcome Trust. The service should help streamline the process of applying for and accessing UK Health Data, including NHS data. This will include having a single point of contact for accessing health data. We have consistently called for a unified national system, streamlined governance, and user-centred technical design – recommendations that should now be realised in the HDRS. We're thrilled to have played a part in this development for members.
For those unfamiliar with the UK health data scene, it sounds like a sensible and unexciting concept. But for those who have grappled with duplicative and time-consuming access processes - which sometimes take months to years to grant access - this announcement is significant. So, can we really solve the UK’s health data problems with this service?
From Goldacre to Sudlow: How did we get here?
For decade’s we’ve seen potential in the UK’s health data for two primary reasons:
- The NHS’s cradle to grave healthcare system, which has produced 70 years of care data.
- Our investment and research advantage in genomics, which has resulted in well over 1 million genomes (with varying levels of detail -whole genome or exome) having been sequenced in the UK by April 2025.
However, complex governing and legal frameworks, alongside a series of high-profile NHS data missteps have slowed progress, such that any strategic advantage in these areas was at risk. BIA have long called for improvements to the data access ecosystem, citing technical and governance challenges as holding back innovation in the sector.
In 2022 BIA published a report, developed by our techbio community: ‘Driving growth and patient benefit through SDEs’ to outline the UK life science SME perspective on the technical and governance requirements for new Secure Data Environments (SDEs). These new infrastructure were called for by the Goldacre Review, followed by the (then Conservative) Government’s Data Saves Lives strategy which sought to address public concerns by shifting to a data access by default model. In this model, data would be accessed in a secure environment, rather than shared by copying data. This new model would increase security, reduce bureaucracy and increase public trust in data access.
Our report and accompanying blog saw these environments as key to streamlining data access, providing they took a user-centred approach, tackling the technical, contracting and governance challenges of those users in the life science sector.
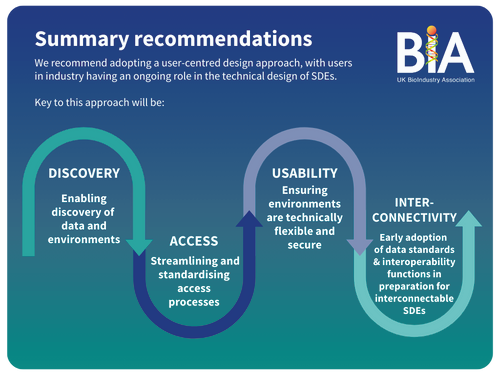
But challenges remained, and in March 2023 the Sudlow Review was commissioned. In our response the review, our message remained consistent, we called for:
Streamlined governance
- A National system - a unified system with a single access point.
- Standardised SLAs - clear service expectations, decision-making transparency, standardised application processes, and agreements.
- Accreditation - similar to data safe haven authentication, accrediting users and organisations should streamline access.
Technical assurance
- Uniformity of technical abilities and security - SDEs should support innovation and offer clear SLAs outlining technical services.
- Interconnectivity - standard data models, interoperability, and data search across all SDEs.
- Standardised information and KPIs - clarity on data availability, uses, and limitations.
In May 2024 the concept of a national data trust was suggested as a mechanism for bringing private sector dynamism to NHS data, attracting media attention.
Then in November the Sudlow review on Uniting the UK’s health data was published. It addressed many of our asks by recommending a ‘national health data service’ which would oversee a ‘national access system’ along with streamlined governance and access. SDE accreditation and a UK wide data strategy would address some of the technical challenges. The Review also rightly called for BIA to be a part of the advisory group for such a service.
A health data research service: what’s the SME perspective
Now the HDRS has been commissioned, we must focus our attention on getting it right. Funding for the HDRS is from the Wellcome Trust and the Government, with plans under development over the coming year. The upcoming Sector plan and Spending Review will contain more detail on Government’s contribution. BIA are actively involved in discussions with the office for life science on these plans.
Robust, thorough and consistent public engagement – including the merits of industry data access – is vital to the running of the programme. Building on that foundation should be comprehensive engagement with users, including those small and scaling companies in the life science sector. Our message has been consistent: addressing the governance and technical challenges through user centred design is vital. We are calling for industry representation on advisory groups of data assets to achieve this. Finally, we have proposed ringfenced funding mechanisms to support small UK life science companies access the UK’s health data. Support UK innovators through timely access to quality health data and the returns will great: better, faster healthcare innovation for patients and the public and jobs and growth for the UK economy. This investment offers a unique opportunity to boost the sector; let’s work together to get it right; for the benefit of everyone in the UK.
- Contact Dr Emma Lawrence to find out more.
- Read: Driving growth and patient benefit through SDEs.
- Read: BIA Submission to Sudlow review on unifying health data in the UK | BIA.
- Join the TechBio Community.




