Driving growth and patient benefit through SDEs
A UK life science SME perspective on technical and governance requirements
The life sciences industry is a key pillar in the UK’s innovation economy. Small to medium enterprises (SMEs) are critical to the growth of the industry and the economy; they turn our world-class academic insights into treatments for patients and products to export internationally. There are 6,548 life sciences businesses in the UK, which employ 68,900 people and generate £8.1 billion of turnover. 77% of these are SMEs which sit at the cutting edge of research and innovation. They are developing new tools and technologies that will revolutionise healthcare, save lives, and improve our health and wellbeing. They are, however, smaller and less resourced than large pharmaceutical companies, meaning they need special consideration by government and other organisations when designing policies and research infrastructure.
Global life sciences are increasingly reliant on access to health data for a variety of purposes, including drug discovery, safety testing, patient stratification and diagnostics development. Despite the importance of health data to life science research and innovation, the process of applying for access, and the data itself, is suboptimal and fragmented in the UK. Key industry requirements for health data have been published by the Association of British Pharmaceutical Industry (ABPI) and the Medicines Discovery Catapult and include data breadth, depth, and scale; speed of access; data quality; expertise; public trust; and affordability.
All these needs can be facilitated by the appropriate use of secure data environments (SDEs), also called trusted research environments (TREs). SDEs are controlled environments where sensitive data can be accessed and analysed without the need to move or copy data into the researcher’s data system. Well-designed SDEs can streamline data access while maintaining data security and privacy. The use of SDEs is particularly important in reassuring the public on data use where there are differing attitudes to commercial research amongst the wider public. They are also a proven model, having been shown to work successfully through infrastructure like SAILDatabank (Wales), the Scottish Data Safe Haven (Scotland) and Genomics England.
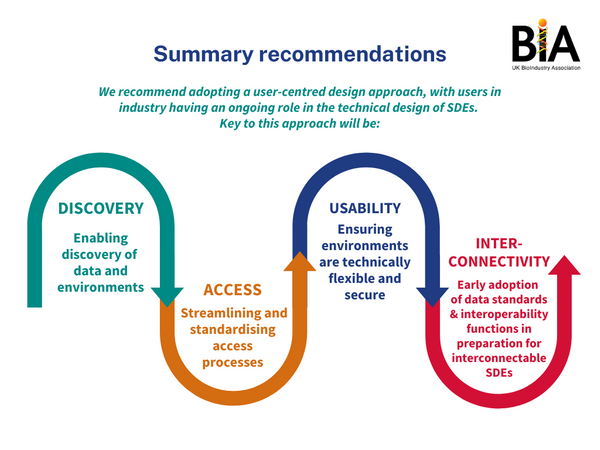
Both, the public and SMEs, are aligned on the need for ways to do life-saving research while protecting the privacy and respecting the choice of individual patients. SDEs support secure, trustworthy, and controlled access to data. As such, industry is supportive of them, provided they facilitate data access in a practical, secure, and timely way. The recent Goldacre Review as well as previous publications heralded SDEs as an answer to many of the concerns raised about health data access. However, the review did not cover industry access in detail.
Furthermore, SMEs face unique challenges in accessing health data, as they have more limited resources to navigate complex governance processes. These innovators also require greater consideration and resources from government and other data custodians to enable the full economic potential of research on genomic and health data to be realised. The move to SDEs presents an opportunity to address many issues associated with data access. This paper sets out what SMEs want to see from the new NHS SDE ecosystem and as such complements the findings of the Goldacre Review and other recent data strategies. To fully realise the Government’s Life Science Vision and further grow the UK’s thriving data economy, use of SDEs should involve industry engagement. The suggestions made in this paper are for stakeholders involved in the development of NHS SDEs. However, insights will also be valuable for those in academia, industry and the charity sector.
This document was informed by the insight of members of the UK BioIndustry Association (BIA) - COHESION Medical, Jiva.ai, PrecisionLife, Benevolent.ai, Congenica Ltd, Human Centric Drug Discovery - but does not represent companies’ opinions and is published by the BIA.






