Celebrating the UK’s progress in its journey to the forefront of RNA manufacturing

When comparing the UK’s RNA manufacturing capability now, to that of before COVID-19, it is obvious that great advancements have been made. Abby Clark, Senior Manufacturing Programme Executive at the BIA, reflects on the past (nearly) five years and highlights recent work in the area.
As COVID-19 unfolded into a global crisis, the urgency for swift action in the UK was undeniable. With a strong foundation in medicine manufacturing, the UK recognised that rapidly embracing new vaccine technologies would be essential in safeguarding lives and strengthening resilience for the future. Academics and researchers worked to understand the most applicable mechanism of action for COVID-19 defence and mRNA therapeutics were unveiled as one of the likely winners - mRNA was, and still is, a new modality which meant existing platforms were not in place ready to manufacture and distribute to the population upon successful process development.
The BIA recognised the difficult challenge ahead and established an industry-led Vaccine Manufacturing Taskforce to support the vaccine candidates from Oxford University (Adenovirus vaccine vector) and Imperial College London (RNA). By drawing on the extensive capabilities within the UK’s biotech sector - home to both academic and industry leaders in biomanufacturing - the BIA leveraged a network of potential expertise to accelerate vaccine development and scale-up manufacturing. However, at the early stage of the pandemic, the UK did not have a centre licenced to manufacture such cutting-edge technology as mRNA or the industry experience and infrastructure. As a result, the UK turned elsewhere for mRNA vaccine supply.
Reflecting on the past 5 years, it's remarkable to see the strides made in RNA therapeutics. The mRNA technology that rapidly delivered a COVID-19 vaccine is now demonstrating exciting potential as a personalised therapy for cancer, highlighting the diversity and complexity of this promising modality. mRNA Covid vaccines deliver the instructions to a cell to create the spike protein found on the virus. The immune system then recognises the protein as a pathogen and induces an immune response to attack the protein and produce antibodies for future viral infection. The technology can be applied to treatment and prevention of cancer; a tumour sample is taken and screened for unique neoantigens (tumour-specific antigens). The neoantigens selected are then coded for within the mRNA and delivered to the patient. The body initiates an immune response and T-cells will in-turn target the tumour cells. The presence of neoantigens is specific to only the tumour cells, so unlike other cancer therapies, healthy cells remain unharmed.
Today, the UK is home to three advanced RNA manufacturing sites; CPI RNA Centre of Excellence, eXmoor Pharma, and Moderna. To showcase these advancements, the BIA has launched an mRNA explainer that highlights the UK’s strengths in manufacturing, research, development, and training. This resource includes case studies from leading companies and provides an overview of the expertise driving innovation in RNA therapeutics across the UK with recommendations on how to sustain the technology by training the next workforce.
Minister of State for Science, Research and Innovation, Lord Vallance, commented on the BIA’s mRNA explainer landscape map:
Thanks for sending through this really interesting and useful piece of work. These sorts of maps and analysis of what we have (and what we don’t have) will be very helpful. As you know, they certainly were during Covid.
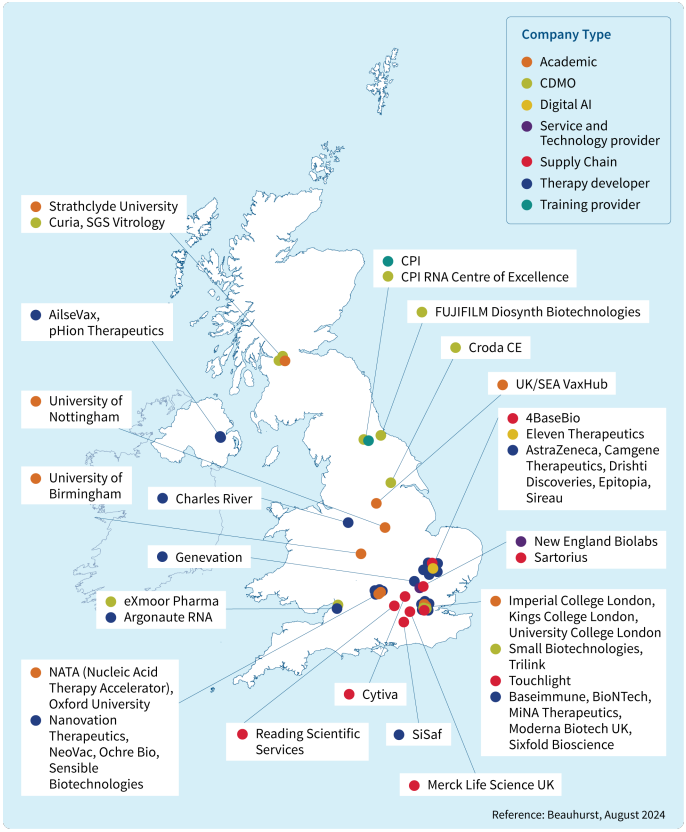
The updated explainer was launched at the RNA Vaccines and Therapeutics Conference 2024, a collaborative event hosted by the BIA, CPI and Imperial College London. The sold-out event was a reflection of the rapid growth and interest in the RNA therapeutics sector. Bringing together 200 professionals from across the industry, the conference provided a platform for experts to exchange insights, discuss advancements, and explore the future potential of RNA therapies.
The UK must build and retain a competitive advantage in RNA manufacturing to continue the growth and resilience already exhibited over the past five years. Our capabilities in this promising strategic technology have expanded significantly in a short space of time. This is a success clearly demonstrated in our report which is worth celebrating as we look ahead to further advancements and leadership in RNA therapeutics.





