bioProcessUK insight: Reimagining Antibody Manufacturing

Ahead of the 19th annual bioProcessUK conference (15-17 November, Edinburgh), which will look at the latest biotech innovation and growth in the UK, including continuous process manufacturing, read our latest blog by Nick Hutchinson, EngD, Business Steering Group Lead (Mammalian Cell Culture) at FUJIFILM Diosynth Biotechnologies. At the conference, be sure to attend the session “Growing the UK Biomanufacturing Footprint” which includes Jonathan Haigh, PhD, Vice President, Process Development at FUJIFILM Diosynth Biotechnologies.
Reimagining Antibody Manufacturing
Developing biopharmaceuticals is very expensive and can take over a decade. Companies that can manufacture their products efficiently once commercialized can maximize their profit margin. Higher profit margins ensure companies can generate greater returns on their investments in drug development. They also provide greater flexibility allowing companies to defend their market share against biosimilar competition in the latter stages of the product lifecycle.
Many companies choose to manufacture mammalian cell culture-derived products using fed-batch technology. Production can be very efficient with this type of technology in large-scale stainless steel manufacturing facilities. However, ramping-up production in this type of facility may require additional investment. This investment includes the cost of chromatography resins and membranes, which are significant. Companies must make this investment before they receive regulatory approval and before they know the likelihood of commercial success. Where process-specific facility modifications are required then additional capital expenditure may be needed. These costs are often worthwhile especially when the demand for a product is high.
Adopting manufacturing strategies that avoid these upfront costs can be strategically beneficial in particular contexts. Companies that have uncertain demand forecasts may wish to consider alternative approaches that avoid putting upfront capital investments at risk. Those firms with low demand forecasts, for example where niche markets are being addressed, may want to avoid the scale-up costs to improve manufacturing economics. Smaller-scale manufacturing strategies using single-use technologies are valuable in these contexts because they can be rapidly and flexibly deployed in response to fluctuations in market demand. The challenge becomes one of improving the productivity of these smaller-scale manufacturing systems to regain some of the efficiencies lost when moving away from large-scale production systems.
FUJIFILM Diosynth Biotechnologies has addressed this challenge by developing a continuous cell culture manufacturing platform called MaruXTM. Continuous manufacturing is more efficient than fed-batch manufacturing at the same production scale. It can also improve product quality, especially for products that are not stable. The platform uses single-use bioreactors run in perfusion and a fully single-use connected downstream process. It is ideal for the manufacturing of monoclonal antibodies, however, it can also be used by customers with other recombinant proteins derived from cell culture. The MaruX manufacturing platform is being implemented into GMP facilities from 2024 at the 500-L scale. The first MaruX projects are entering process development this year.
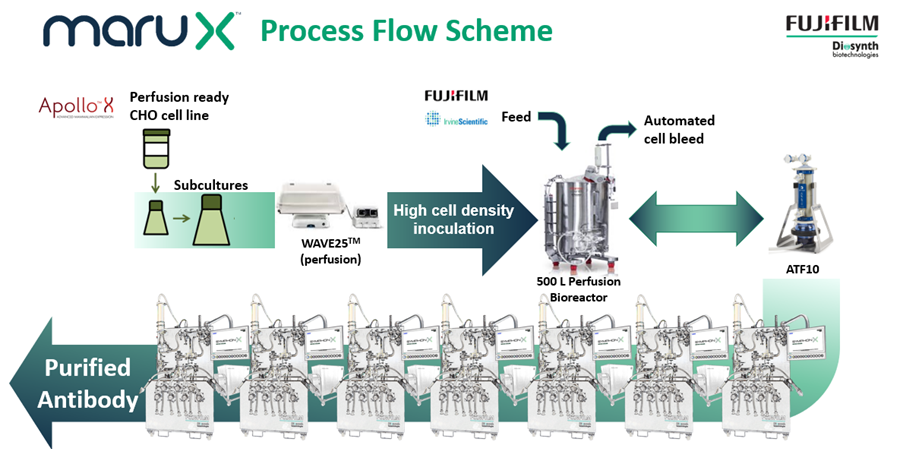
FUJIFILM Diosynth Biotechnologies’ MaruXTM platform for the continuous manufacturing of antibody and other biopharmaceuticals from mammalian cell culture
We expect a typical MaruX process development program to take only two months more from the start of cell line development through to the release of drug substance than a standard fed-batch program would. The total costs for a program that will allow a client to reach their Investigative New Drug (IND) submission will be no more than those for a fed-batch program. The benefits will be realized when clients need a resupply of material for clinical trials.
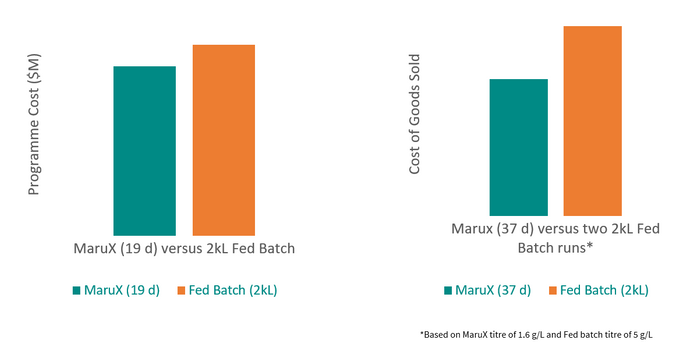
The cost of MaruX programme from cell line development to IND-submission is lower than for a standard fed-batch programme but can reduce manufacturing COGs by 28%
The full benefits of the MaruX platform will be seen once products enter commercial manufacture. Multiplexing 500-L or 2000-L single-use bioreactors together and purifying the perfusate from each simultaneously has the potential to supply hundreds of kilograms of products to the market very efficiently. Leveraging this unique technology will allow clients to maximize their production efficiency while retaining the agility and flexibility engendered by single-use manufacturing technologies.
Five Key Factors to Consider When Choosing Continuous Bioprocessing
- Niche product or blockbuster?
Continuous manufacturing in single-use systems is ideal for niche or orphan products. Mega-blockbusters required in multi-tonne production each year is better suited to large-scale fed-batch production in stainless steel tanks.
- Uncertainty in demand forecast?
Biotech companies can avoid high upfront costs associated with large-scale manufacturing where demand forecasts are uncertain due to the flexibility of continuous manufacturing in single-use systems.
- Product stability?
Continuous manufacturing is ideal for labile products. The residence time of the molecule in the bioreactor is significantly reduced and it is rapidly purified from the harvest pool.
- Product quality attributes?
If it is important that a candidate must have specific quality attributes such as high galactosylation, low aggregation or low levels of acidic species, continuous manufacturing might provide the best manufacturing solution.
- Protein expression levels?
Firms with difficult-to-express proteins should consider continuous manufacturing to take advantage of greater productivity associated with perfusion cell culture.
Learn more about FUJIFILM Diosynth Biotechnologies and meet them at the 19th Annual bioProcessUK conference.





