Beating the bottlenecks: unleashing AI on cell and gene therapy

Innovating manufacturing processes is key to revolutionising access to advanced cell and gene therapies - this blog explores how Tolemy Bio's co-founders, Caelan and Alex, are leveraging cutting-edge technologies like AI, biochemical modelling, and omics data to overcome complex challenges and drive global scalability.
Ten years ago, Emily Whitehead’s remarkable recovery from an otherwise incurable blood cancer marked the dawn of a new era in medicine. CAR-T cell therapy saved her life and opened the door to treating countless conditions once thought untouchable. Yet despite this milestone, fewer than 3% of eligible patients have gained access to these transformative therapies. Manufacturing complexities - scaling up production, maintaining consistent quality, and ensuring cost-effectiveness - continue to constrain the field. To make these breakthroughs widely accessible, we need new strategies that go beyond incremental improvements. One such approach lies in optimising the very environment where these cells grow: the cell culture media.
A growth field, constrained by manufacturing
Cell and gene therapies are expanding rapidly, with potential applications ranging from haematological cancers and autoimmune diseases to regenerative treatments after heart attacks. But the challenge is clear: while the science of reprogramming immune cells to fight disease has matured, the processes that convert a personalised cell line into a scalable, robust therapy have not kept pace. Autologous CAR-T production is notoriously slow, expensive, and prone to variability. Emerging allogeneic approaches, and promising off-the-shelf treatments, face their own hurdles in ensuring that cells expand efficiently, maintain the right phenotype, and meet stringent quality controls.
This gap between promise and practice means that too many patients still cannot access these therapies. The next frontier is manufacturing innovation, and one of the most critical levers is the cell culture environment.
Media: the unsung hero of cell manufacturing
Within this complex manufacturing landscape, culture media may seem like a small detail - but it plays an outsized role in determining the fate of cells and cell-based products. The right media formulation can dramatically improve cell growth, enhance therapeutic potency, and fine-tune specific attributes like cytotoxicity and persistence. Some studies have shown that simply changing media composition can drive 100-fold increases in cell density. In a field where yield, consistency, and function are paramount, media holds the keys to unlocking higher-quality therapies at scale.
Yet, despite its importance, the industry currently relies on a handful of generic, off-the-shelf media formulations. These formulations assume that every cell therapy - regardless of patient population, disease target, or cell lineage - has the same metabolic needs. This “one-size-fits-all” approach doesn’t just miss opportunities; it actively constrains therapeutic potential.
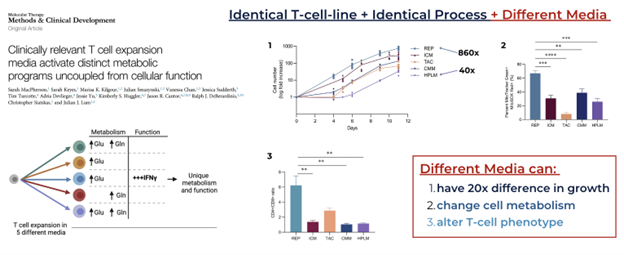
Figure 1: Study showing the effect of media on T cells across metabolism and phenotype
Why media optimisation is so hard
If optimising media were simple, it would already be standard practice. The reality is that formulating an ideal culture environment is a monumental challenge:
- Combinatorial explosion: There are thousands of potential ingredients and concentrations. Adjusting just a handful can produce millions of possible combinations.
- Biological complexity: Different cell types, disease indications, and manufacturing goals require unique metabolic states. A formulation that enhances one product attribute might diminish another.
- Non-linear responses: Cellular behaviour doesn’t scale neatly with ingredient concentration. A subtle change in micronutrient levels can produce large, unpredictable shifts in cell function.
Traditional approaches, like Design of Experiments (DoE), sample the space in a linear, brute-force manner. They can identify basic ingredients but struggle to uncover complex interactions or identify the subtle metabolic cues that drive optimal cell states. As a result, most manufacturers stick to the standard options already on the market, leaving significant gains on the table.
A smarter approach: model-driven media design
Traditional methods for media optimisation often imagine cell metabolism as a gently sloping plain - if you travel steadily north, for example, they assume the landscape will rise or fall at a predictable, even gradient. In reality, biology isn’t so straightforward. Instead of smooth hills, you’re navigating a rugged, twisty mountain range with unexpected cliffs, hidden valleys, and winding paths. By ignoring these complexities, conventional approaches rely on a crude “paper map” where much of the terrain is left blank, forcing scientists to rely on guesswork rather than guided exploration.
What if, instead, we could capture the true contours of this metabolic terrain - filling the map with hills, valleys, and winding pathways that reflect genuine biological processes? This is where a model-driven approach comes in. By integrating core biochemical data (like essential nutrients and energy sources) with deeper omics insights (signals, differentiation cues, and subtle metabolic interactions), we produce a richly detailed, topographical map of the cell’s metabolic world.
This new, biologically accurate map eliminates the need for random trial-and-error or biologically implausible guesses. Armed with advanced AI and machine learning - think of it as “Google Maps” for cell culture - we can navigate these metabolic landscapes, adjusting specific factors with confidence. The result? Multi-objective optimisation that improves not only cell growth and expansion times but also therapeutic potency, persistence, and consistency. Instead of inching forward blindly, we can chart the best possible route through complex metabolic territories, ensuring that the cells - and ultimately, the patients - reap the benefits.
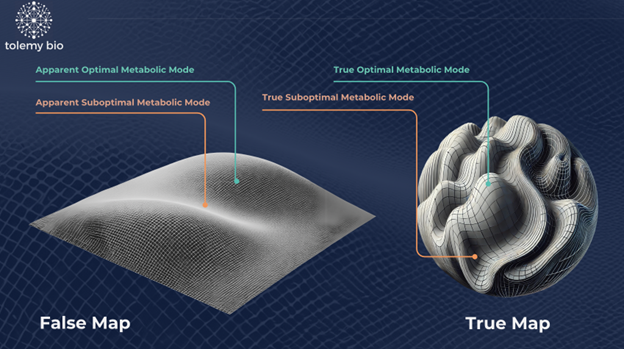
Figure 2: Visualisation of the media landscape, comparing traditional methods to those with AI
Real-world impact and the path forward
This model-driven approach is not just theoretical. Early collaborations - with research institutions, technology groups, and bioreactor manufacturers - are already validating how tailored media can streamline manufacturing for diverse cell therapies. At Tolemy Bio, for example, we’ve partnered with MFX, a next-generation bioreactor platform manufacturer, to apply our model-driven media strategies specifically to T cells. By integrating our biologically rich, data-informed formulations into MFX’s automated systems, we’re eliminating guesswork, reducing scale-up times, and driving better consistency in T-cell production. The result is a faster, more reliable pathway to bringing potent, patient-ready cell therapies to the clinic.
As allogeneic therapies and new modalities like CAR-NK or iPSC-derived products become more prevalent, the complexity will only grow. However, with model-driven media optimisation, we can turn complexity into an asset. Instead of being paralysed by the enormity of the search space, we can leverage it to discover better solutions faster.
Conclusion
Improving manufacturing is critical to making advanced cell and gene therapies accessible on a global scale. While media optimisation might have once been viewed as too complex to tackle, the integration of biochemical modelling, omics data, and AI-driven insights makes it possible. By designing custom media formulations that align with each product’s unique metabolic needs, we can help usher in a new era of scalable, efficient, and broadly available cell therapies.





