Introduction
Welcome to our new-look quarterly report, where the BIA team share examples of the influence we are delivering for members on government policy and the global life sciences ecosystem.
As the new government settled in and Parliament returned from summer recess, BIA’s engagement activities went up a gear in the fourth quarter of 2024, with our flagship influencing event, Parliament Day, giving members an unrivalled opportunity to meet with new policy makers in Whitehall and Westminster. We also represented members at the first meeting of the Life Sciences Council under the new government and established a new group of MPs and peers to champion life sciences in Parliament.
Life sciences was selected as one of the eight great sectors to drive growth in the new industrial strategy, with BIA helping produce a new sector plan with government. A House of Lords report adopted many of BIA’s recommendations on how to support engineering biology businesses and a major independent review of health data recommended BIA be on a new industry advisory board for government. We also averted a new tax being imposed on the sector.
We published data showing equity investment in the sector growing and the first London Life Sciences Week brought the global sector and investors to the capital in November, cementing the UK’s position as the pre-eminent life sciences hub outside the United States.
Read on for more insights from the team.

Government and Parliament
Parliament Day offered exclusive platform for BIA members to engage parliamentarians on key issues
On 14 November, the BIA hosted its 24th annual Parliament Day, where 38 representatives from BIA member companies met with 26 senior stakeholders across Westminster and Whitehall. For BIA members, the event delivered unparalleled access to decision-makers, ensuring the sector’s priorities are at the forefront of government strategies, supporting growth, innovation, and the UK’s global life sciences leadership. The day came at a pivotal time, with a new Labour Government highlighting the life sciences sector in their first Budget and the Industrial Strategy Green Paper.
The day featured discussions with Shadow Secretary of State for Science Alan Mak, members of the House of Commons select committees scrutinising the Government’s work on Health, Science, Innovation and Technology, as well as senior civil servants from key departments. Members shared their priorities, such as:
- unlocking pension fund investment for scaling biotech companies
- enhancing R&D tax relief for growth sectors
- fostering a pro-innovation NHS for rapid adoption of new treatments.
Another highlight was the meeting with Dr. Dave Smith, National Technology Advisor at DSIT, where members gave insights into the technologies they are bringing to market. Parliament Day reaffirmed the BIA’s influence in shaping life sciences policy, strengthening the sector's voice, and fostering meaningful relationships with policymakers.
BIA partners with the third sector to give the life sciences a stronger voice through the re-launched APPG
The All-Party Parliamentary Group (APPG) for Life Sciences was officially reinstated in October, after merging with the APPG on Medical Research. BIA provides the secretariat in partnership with industry colleagues, leading medical research charities and the UK's National Academy for medical sciences.
Chaired by the Rt Hon Kit Malthouse MP, the re-formed APPG marks a significant step in elevating life sciences within the political agenda as, for the first time, parliamentarians have direct access to the entire life sciences ecosystem. This coordinated, joined-up advocacy approach promises to benefit both industry and the third sector, giving us a stronger chorus to urge greater government focus on backing the life sciences.

Kit Malthouse MP, Pippa Heylings MP, Steve Yemm MP, Baroness Finlay, and Sadik Al-Hassan MP at a recent APPG planning meeting
As a first action, the APPG wrote to the Chancellor urging her to protect and promote the sector in the government's inaugural Budget. They highlighted that industry and medical research charities collectively support over 326,000 jobs, and in 2023 invested £9 billion and £1.7 billion in R&D respectively. The APPG also runs topical events in Parliament and sends a regular news bulletin to continue raising all parliamentarians’ awareness and understanding of the valuable contribution the life sciences provide to the health and wealth of the nation.
The BIA leads the APPG secretariat function on behalf of the life sciences industry with the Association of Medical Research Charities (AMRC) and with support from Cancer Research UK (CRUK), the Association of the British Pharmaceutical Industry (ABPI), The Academy of Medical Sciences, Versus Arthritis, The British Heart Foundation and the British In Vitro Diagnostic Association (BIVDA).
Life Sciences Council meets for the first time under Labour

Cabinet Ministers Wes Streeting and Peter Kyle were flanked by Gillian Merron and Patrick Vallance, while Emma Walmsley of GSK chaired from the industry side. Source: Gov.uk
The top decision-making group for life sciences industry-government partnership working met for the first time under the new Labour government on 22 October. Chaired by Health Secretary Wes Streeting, Science Secretary Peter Kyle, and GSK CEO Emma Walmsley, it was a key moment for both sides to understand each other’s priorities.
BIA was represented by CEO Steve Bates with our guest member Bicycle Therapeutics’ CEO Kevin Lee.
Both made a strong case for more to be done to increase access to finance for UK scale-ups and support to secure medicines manufacturing facilities in the UK. Industry’s priorities for health data, clinical trials and the commercial environment were also shared by representatives around the table.
I was delighted to be invited by BIA to represent scaling UK biotech at the first Life Sciences Council meeting with the new government. Membership of BIA gives us a strong united voice, and opens opportunities to meet with policymakers to support our sector.

Streeting and Kyle reiterated the government’s support for the sector, and strongly referenced Labour’s pre-election Prescription for growth document, which BIA helped produce and launch in February.
Among the outcomes of the meeting were for task and finish groups to be established on the priority areas set out by industry to inform the new industrial strategy and sector plan.
BIA outlines growth priorities for the life sciences in response to the Industrial Strategy
The BIA submitted a response to the Department for Business and Trade's consultation on Invest 2035: The UK's modern industrial strategy. The strategy is set to outline the government’s 10-year plan for both the stability and certainty needed to encourage investment in the UK’s priority growth sectors.
The government designated the life sciences as one of the key growth-driving sectors that the strategy will focus on, a decision warmly welcomed by the BIA. To capture a greater share of internationally mobile investment in life sciences and grow the UK sector, we advised that the strategy should strengthen existing partnerships between industry and government, such as the Life Sciences Council. From these partnerships, government should continue to advance policy priorities in key technologies and subsectors – engineering biology, deep biotech, genomics, mRNA, cell and gene therapies, and precision medicine – while addressing the most significant barriers to growth, such as access to scale-up finance, skills, regulation and trade disruption. Many of the policies to achieve this will be set out in a life sciences sector plan under the new industrial strategy.
BIA and members help shape new sector plan
In December, the Office for Life Sciences established multiple task and finish groups and an overarching advisory group to develop the new life sciences sector plan.
BIA secured member representation on most of these groups, with CEO Steve Bates taking a seat on the advisory group, which will assess the task and finish group’s recommendations to form the plan.
BIA’s Chair, Dan Mahony, is leading the access to finance group. The Chair of our Data, AI and Genomics Advisory Committee, Steve Gardner, is representing SMEs on the Data group and the Chair of our Rare Disease Industry Group is on the Access and Uptake group. BIA also secured a place for Elin Davies, CEO of Aparito, on the R&D group.
The task and finish groups will produce their final recommendations in February to inform the sector plan, which is expected to be published in late May, or early June, alongside the industrial strategy, 10-year health plan and spending review.
BIA highlights members’ role in transforming healthcare through innovation in the NHS 10-Year Health Plan consultation
The BIA responded to the Government’s NHS 10 Year Health Plan for England consultation. In our response, we called for continued partnership working with the life sciences sector, who will be key players in the shift to prevention through early diagnosis and the better use of technology in health and care. The role that critical technologies such as cell and gene therapies, techbio, and precision and genomic medicine will play in creating an NHS fit for the future was also highlighted. In addition, former BIA Chair Ruth McKernan has a role on the Research, Innovation and Science Working Group for the plan, further amplifying the BIA’s voice.
First London Life Sciences Week shines spotlight on the UK sector
BIA organised the first London Life Sciences Week in November in collaboration with London and Partners, part of the London Mayor’s Office.
Thousands of global investors and companies assembled in London during the week that includes the annual Jefferies Healthcare Conference and its many satellite events, all under the banner of London Life Sciences Week.
On the Wednesday, BIA and the British Business Bank hosted an exclusive breakfast in the House of Lords, chaired by Lord Willetts, where Science Minister Lord Vallance gave a keynote speech reinforcing the government’s commitment to improving access to finance.
Later that day, the government announced that BIA’s Chair, Dan Mahony, was reappointed as the government's Life Sciences Investment Envoy, a role he had under the last government. Dan will champion start-ups and scale-ups with the support of a team in the Office for Life Sciences.
Business environment
Finance and tax
BIA wins commitment from new Chancellor to protect R&D tax reliefs
As a result of BIA's sustained campaigning, Rachel Reeves announced at the International Investment Summit that Labour will "maintain the rates of relief for R&D, which provide generous support for innovation." Shortly before this announcement, Steve Bates OBE, CEO of the BIA, led an initiative with 150 industry CEOs, who signed a letter to the Chancellor with this demand.
This commitment – achieved through BIA’s close relationship with the Treasury over many years – reinforces the strong foundation for the biotech sector’s future growth, and was echoed in the Chancellor’s highly anticipated Autumn Budget, which put life sciences at the heart of the government’s growth mission and delivered reassurance and stability to the sector.
In addition to reaffirming the commitments to the reliefs made at the Investment Summit, the Budget included acknowledgment from HM Treasury of the frustration that genuine companies experience regarding HMRC inspectors – an issue BIA has raised repeatedly. HM Treasury said it will be “enhancing the administration of R&D reliefs” by establishing the R&D expert advisory panel (announced by the previous government), continuing to improve signposting and guidance on R&D reliefs, and launching an R&D disclosure facility by the end of 2024.
Finally, the BIA’s call for a review of R&D tax relief and its support for life sciences also received a reference: "The government is committed to periodically evaluating the R&D reliefs to ensure they are as effective as possible and underpinned by a credible, up-to-date evidence base."
This review is crucial to the accurate valuation of the reliefs within the life sciences, especially in the face of increased levels of non-compliance and anticipated government action.
This commitment from government to maintain the rates of R&D tax relief for the duration of Parliament represents the culmination of years of the BIA’s government influencing work. It is a vital step for the growth of our sector, and the BIA will continue working to ensure that the programme is fit for purpose.
BIA meets with Tax Minister to discuss the future of R&D tax reliefs
In light of the government’s commitment to R&D tax relief, the BIA met with the Exchequer Secretary to the Treasury, James Murray MP to discuss future priorities for the newly protected and recently reformed R&D tax relief scheme, with a focus on ensuring that the reliefs remain fit for purpose.
BIA shared members’ views that the administration and reputation of the schemes must be considerably improved to maintain the UK’s standing as a location for innovation. The Minister agreed to work in partnership to help HMRC enable legitimate and eligible companies to claim as efficiently and smoothly as possible.
Government announcements show pension reform progress as Chancellor visits BIA member
In November, the Chancellor delivered her first Mansion House speech committing the government to the radical reform of UK pension funds to support growing businesses in the UK – including life science companies - a key, long-term objective of the BIA. Ahead of the speech, the Chancellor met with our CEO, Steve Bates OBE, at BIA member company Quell Therapeutics, underlining her support for the sector.

Rachael Reeves and Emma Reynolds at the Quell Therapeutics Translation & Innovation Hub with CEO Iain McGill and team. Source: Quell Therapeutics
Further announcements came alongside the speech. The British Business Bank outlined how pension providers Aegon UK and NatWest Cushon have committed a cornerstone investment to the British Growth Partnership. The British Business Bank also announced a new fund under a joint initiative with Schroders Capital, the $97.3 billion specialist private markets business.
This is the first ever long-term asset fund (LTAF) dedicated to UK venture capital, which Schroders will begin investing before the end of the year, with 20% of the fund expected to be invested in life sciences. In addition, the Pensions Investment Review interim report was published.
The report reflected the detailed and evidenced policy case the BIA has long been presenting, namely that ‘The government is concerned by the evidence that UK pension funds are investing significantly less in the domestic economy than overseas counterparts.
Following these announcements, the BIA met with the CEO of British Business Bank, Louis Taylor to discuss the British Growth Partnership in more detail. From this meeting, a plan is in place to host a roundtable bringing together the British Business Bank with UK life science venture capital firms to understand what the British Growth Partnership will mean for the sector.
BIA and ABPI push for UK CROs to be supported in R&D tax relief regime
The BIA worked together with the ABPI to write and submit a briefing to government outlining the life science industry’s concerns regarding the R&D tax incentive regime’s unclear position on contracted R&D undertaken by UK service provides, such as Clinical Research Organisations (CROs), which is undermining the UK’s competitiveness and ability to attract global life sciences investments that will grow the economy. If left unresolved, this uncertainty risks disincentivising overseas companies from contracting UK companies to deliver research and manufacturing services, which would hinder the government’s missions to drive economic growth and build an NHS fit for the future. HM Treasury and HMRC agreed to meet in the new year to resolve the issue.
BIA shares insights into the effectiveness of advance clearances
In the Budget, the Government committed to a discussion with stakeholders around the widening of advance clearance usage in R&D reliefs and plan to launch a formal consultation on the various options in Spring 2025. Advanced clearances are available to businesses who wish for HMRC to provide a provisional assessment of a company’s eligibility to claim R&D tax reliefs.
Ahead of the formal consultation, the BIA and our R&D tax relief subcommittee met with HM Treasury and HMRC to share insights into the current state of the initiative, and the nature of its usership. Amidst a productive conversation, the BIA called for an expansion of eligibility criteria for the clearances, allowing those that have previously claimed tax reliefs to apply. In addition, due to the nature of the life sciences, most companies are aware that what they are doing qualifies as R&D, and so don’t use the programme. To combat this, we suggested different types of pre-clearance for certain sectors that would allow applicants to ask more targeted questions about specific areas of the application that might affect their success.
The UK biotech sector shows encouraging resilience following a strong second quarter
We published our latest UK biotech financing report that documents the encouraging resilience of investment in the sector from July to September 2024.
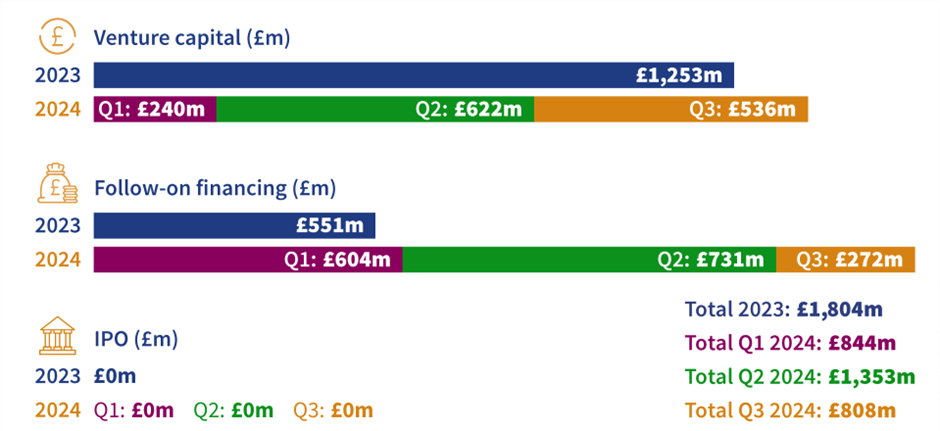
Venture capital investment maintained a solid performance of £536 million across 18 deals. The steady flow of deals highlights continued investor confidence in the sector’s potential to drive innovation and deliver growth. To the end of September, £1.61 billion had been raised in follow-on financing, the largest total in the last five years. While a slight decline was witnessed from the previous quarter, the overall trend for 2024 indicates that the biotech sector continues to attract investment, highlighting its strength and appeal to both domestic and foreign investors.
For further information on the BIA’s work on finance, tax and investment, please contact Lewis Miles, Policy and Public Affairs Manager.
IP and tech transfer
Global tax on UK biotech sector narrowly avoided after BIA campaign
BIA led significant efforts to avert a mandatory levy or tax being imposed on the biotech sector. As the threat to businesses and innovation became clear, BIA undertook detailed discussions with government officials, international partners, and UK ministers, including securing a roundtable with Minister for Nature Mary Creagh MP to hear the views of the sector immediately ahead of the negotiations at COP16.
The detailed engagement with government happened in the run-up to COP16 in November, where the Parties to the Convention on Biological Diversity (CBD), including the UK, established a multilateral benefit sharing mechanism and global fund, the Cali Fund, for the use of Digital Sequence Information (DSI) of genetic resources. The international agreement avoided mandating businesses to pay into the fund. The result, which BIA campaigned for, means that UK commercial users in the biotech, pharmaceutical and other sectors who use DSI accessed from public databases ‘should’ (not must) contribute to the new Cali Fund a percentage of their revenue (0.1% as indicative) or profit (1% as indicative).
The CBD has published four consultations that will further cement the details of the mechanism, and definition and thresholds of companies that are asked to contribute to the Fund. The BIA will be responding, and continue to engage closely on this issue.
BIA members responds to DEFRA’s Nagoya Protocol Post Implementation Review
In December, the Department for Environment, Food and Rural Affairs (DEFRA) invited industry, academic and other stakeholders to share their views on the UK’s implementation of the Nagoya Protocol (Compliance) Regulations 2015 and associated Guidance.
BIA and members responded to the survey. BIA highlighted industry’s concerns over the effects of the regulation on innovation, and detailed certain targeted amendments to the regulations and guidance that will alleviate some of the problems faced by innovators who need to comply with the regulations.
This Post Implementation Review (PIR), conducted every 5 years, is the first opportunity for submitting feedback on the effectiveness and appropriateness of the regulations and guidance since Brexit, and since enforcement activities were undertaken by the Office for Product Safety and Standards (OPSS) in 2022.
To find out more about the BIA’s ongoing work on IP and tech transfer, please contact Senior Policy and Public Affairs Manager Linda Bedenik.
Regulation and access
BIA members help shape the future of clinical trials as landmark legislation laid in Parliament
On 12 December 2024, new legislation was laid in Parliament to modernise the regulation of clinical trials in the UK. This new legal architecture is an important milestone in the Medicines and Healthcare Products Regulatory Agency’s (MHRA) biggest overhaul of the clinical trial regulations in 20 years. The new legislation has been discussed and debated for a while, and the BIA responded to a consultation on the proposals in 2022. In that submission, we highlighted the opportunity to create a world-class regulatory environment for clinical trials and support faster patient access to innovative medicines.
The new legislation will be accompanied by a number of supporting guidance documents, and the BIA is engaging with the MHRA on the development of these. In December, the BIA submitted a response to the informal consultation on the Health Research Authority (HRA) and MHRA’s draft inclusion and diversity guidance for clinical trials, which was developed with input from the BIA Regulatory Affairs Advisory Committee (RAAC). The BIA and our members will continue to engage with the MHRA and HRA as guidance is developed.
MHRA announces plans for ILAP refresh, incorporating BIA members’ feedback
At the BIA Regulatory Conference on 6 November 2024, the MHRA announced a statement of policy intent for the relaunch of the Innovative Licensing and Access Pathway (ILAP). ILAP was launched in 2021 with the aim of accelerating patient access to innovative medicines for patients in the NHS.
The BIA has engaged closely with the MHRA on its plans to relaunch ILAP, helping to identify learnings from our members’ experience of ILAP to date. We welcomed the confirmation that the NHS will be a full partner in the refreshed pathway, enabling ILAP to provide a single integrated platform for collaborative working between the developer, MHRA, HTA bodies and the NHS. We hope that the refreshed ILAP will be able to offer predictable timelines for companies, enabling companies to plan more effectively. The new ILAP will be launched and opened to applications in March 2025.
BIA continues to support preparations for Windsor Framework implementation
Ahead of the implementation of the Windsor Framework on 1 January 2025, the BIA has continued engagement via monthly meetings involving the MHRA, DHSC, and industry associations. The Windsor Framework introduces new rules in the UK for product licensing, labelling, and the EU Falsified Medicines Directive.
It will ensure that medicines can be approved and licensed on a UK-wide basis by the MHRA, and medicines can be supplied in the same packs across the UK. The latest engagement meetings have focused on the final guidance documents published on the Windsor Framework Hub, including the category lists of products. We also fed into the MHRA’s communication plans to encourage marketing authorisation holders to make their labelling submissions before the end of 2024.
MHRA and NICE engage with industry on system alignment
In October 2024, the BIA attended a workshop with the MHRA and NICE to discuss alignment across the UK regulatory and access pathway for medicines. The workshop was focused on how to support faster patient access to medicines through closer alignment between the date of marketing authorisation approval and the publication of NICE guidance. Attendees discussed the potential benefits of joint pipeline meetings with the MHRA and NICE to support better planning, as well as efficiencies which could be derived through information sharing across overlapping evidence requirements.
The BIA welcomed the focus of the workshop on creating more joined-up regulatory and access processes in the UK, and we look forward to continuing to engage with the MHRA and NICE as this work progresses.
For further information on the BIA’s work on clinical trials, ILAP, and medicines licensing, please contact Senior Policy and Public Affairs Manager Rosie Lindup.
Skills and talent
BIA influences skills policy through roundtables with new arms-length body
Throughout the autumn BIA has been engaging with Skills England, a new arms-length body within the Department for Education that will bring together key partners to meet the skills needs of the next decade across England working in partnership with devolved nations. Launched in shadow form in September 2024, with plans to be fully established in 2025, this new body will aim to form a coherent national picture of skills gaps and work closely with Industrial Strategy Council and Migration Advisory Committee to address skills needs and shape the future of education.
In its first report: Skills England: Driving Growth & Widening Opportunities, Life Sciences was identified as a sector of strong growth with robust evidence already available on economic importance and skills forecasting.
BIA and member companies were invited to two virtual roundtables. The first discussed:
- whether their report accurately highlighted key skills gaps and challenges across the sector
- how they can build on our existing analysis
- where to focus how they can help to address these gaps in its first few years of operation
The second roundtable sought further expertise on:
- skills needs in the sector
- skills elements of the Life Sciences sector plan for the Industry Strategy
- advice on how flexibilities in the Growth and Skills Levy could deliver on the aims of supporting economic growth and opportunity
A further report from Skills England is due Q1 2025 containing reflections on their feedback and developing strategies for education policy reform on which BIA will engage.
BIA and KQ Labs propel TechBio startups forward with support from the Government’s Shared Prosperity Fund
The BIA, in partnership with KQ Labs at the Francis Crick Institute, successfully delivered the 12-week TechBio Boost accelerator program, supporting Seed/Series A startups to scale and grow within the London area, and connecting them with investors and other key industry stakeholders. Funded through the UK Shared Prosperity Fund (UKSPF)—a key element of the UK government’s Levelling Up agenda—the program highlights the BIA’s commitment to driving growth and fostering innovation in the UK biotech sector.
The team secured funding from the Mayor of London after presenting their vision to London & Partners and the Grow London team. The program included flagship events such as a US Commercialisation workshop, where members gained exclusive insights from the Department for Business and Trade’s life sciences and venture capital specialists William Lindsay and Jack Conway. Another highlight was the Demo Day at the Francis Crick Institute, which brought together key corporate figures, including Paul Ashley (J&J) and Emily Jefferson (HDR UK). Notably, participating companies such as Jiva.ai are also members of the newly launched BIA Data, AI, and Genomics Advisory Committee, reflecting the synergies between the programme and BIA’s key policy focus areas. Readers can explore the full TechBio Boost cohort, discovering more about these companies and their groundbreaking work, via the TechBio Boost cohort page.
Please contact the BIA’s Skills Strategy Consultant Dr Kate Barclay to learn more about the BIA’s work on people, skills, and talent.
Manufacturing
Science Minister praises new BIA resource that maps the rapidly-growing RNA therapeutics sector
To a sold out audience at the RNA Vaccines and Therapeutics Conference in October, the BIA launched our re-vamped mRNA report, which features a map of UK RNA companies, a timeline of developments and a focus on saRNA. In a later response, Minister of State for Science, Research and Innovation, Lord Vallance, commented:
BIA hosted the conference in partnership with CPI and Imperial College London, offering a platform for 200 professionals to exchange expert insights, discuss advancements, and explore the future potential of RNA therapies. Given the evident interest and rapid growth, BIA will continue to champion the area and follow the latest developments.
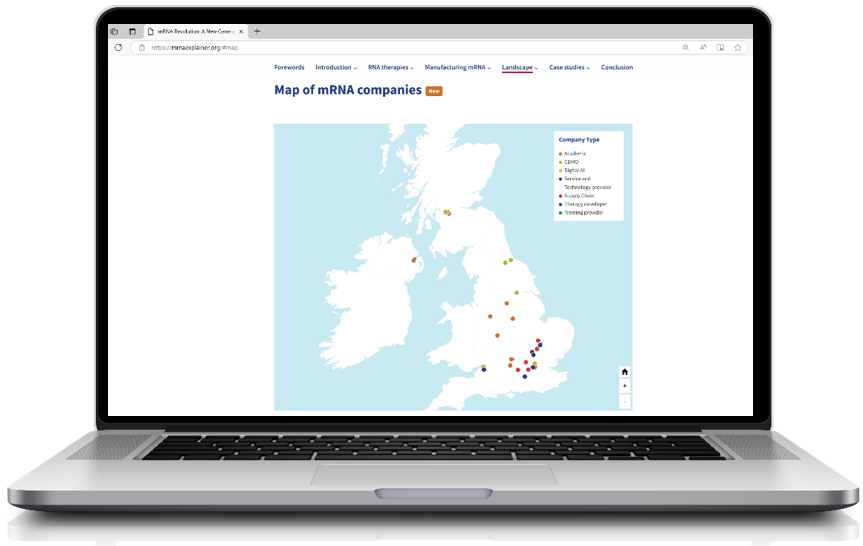
BIA map visualising the extent of mRNA capability across the UK, including SMEs as well as academic and large corporate centres of excellence.
21st bioProcessUK event in Liverpool sparks local action
The BIA's bioProcessUK conference took place in Liverpool for 2024, featuring an engaging programme curated by a subgroup of the Manufacturing Advisory Committee. Key topics included sustainable medicines manufacturing and infectious disease. Attendees heard from Roz Campion, Stella Peace, AstraZeneca GM Mark Proctor, and Purespring CSO Alice Brown during a mastermind-style session led by BIA CEO Steve Bates, which explored recent UK investments in medicine manufacturing.

BIA staff and members with Liverpool City Region Mayor, Steve Rotherham (third from right), at bioProcessUK
In collaboration with the BIA's Cell and Gene Therapy Advisory Committee, a panel discussed ways to enhance the value of cell and gene therapies. Key challenges highlighted for the audience included data collection, storage, and the debate around build versus buy strategies.
The Mayor of the Liverpool City Region, Steve Rotheram, joined delegates at the pre-party event to talk about the importance of medicine manufacturing within the local area. With collaboration and innovation at the core of the bioprocessing sector, the event sparked discussions that will drive future projects and advancements across the industry.
BIA MAC sustainability workstream publishes case studies to share sector Net Zero initiatives
BIA's Manufacturing Advisory Committee sustainability working group have published case studies to showcase how MAC members are championing sustainable ways of working and share knowledge across the sector. The case studies encompass cross-sector challenge solutions which aid our industry in moving towards a Net Zero manufacturing approach and supporting the government’s policy ambitions to be carbon neutral by 2050. Bioprocessing companies are implementing techniques such as process intensification, where processes are streamlined to use fewer materials, energy, and water. By optimising cell culture conditions and nutrient delivery, for instance, companies can achieve higher yields from smaller batches, leading to less resource consumption per unit of product.
BIA's webpage will act as a platform for companies to implement new ideas and keep striving towards a sustainable future.
Please contact the BIA’s Senior Manufacturing Programme Executive, Abby Clark to learn more about the BIA’s work on people, skills, and talent.
Critical technologies
Engineering Biology
House of Lords engineering biology inquiry adopts BIA recommendations for supporting UK SMEs
The House of Lords Science and Technology Committee has published its report, Don’t fail to scale: seizing the opportunity of engineering biology. This report concludes their inquiry into engineering biology, to which the BIA contributed both a written submission and an oral testimony provided by Dr Martin Turner, Director of Policy and External Affairs.
In the submission, BIA urged the Government to seize the opportunity to drive progress during the first half of the Government’s 10-year National Vision for Engineering Biology, underscoring three areas that are essential for the success for UK SMEs: access to infrastructure, supportive financial measures, and regulatory clarity.
The Committee recommends that Government recommit to the £2 billion spending target set out in the National Vision, use the industrial strategy to set out a clear plan to develop engineering biology, and urged coordinated Government action across public and private investment, procurement, skills, regulation, infrastructure and incentives. These recommendations bolster the case for targeted financial and regulatory reforms ahead of the upcoming government spending review and industrial strategy publication.
BIA calls for inclusion of engineering biology and deep biotech as frontier technologies in UK industrial strategy
On 1 October, the BIA wrote to Lord Vallance, Minister of State for Science, Research and Innovation, calling for engineering biology and deep biotech to be prioritised as frontier technologies in the Government’s forthcoming industrial strategy, a request we doubled down on in our industrial strategy submission. BIA argued that these technologies have the potential to drive economic growth while enabling the UK’s net zero and green transition.
The letter highlighted the UK’s strengths in engineering biology, including its world-leading R&D base and thriving startups, which collectively raised over £5.2 billion between 2017 and 2022. However, BIA emphasised the need for coherent government support across regulation, finance, and infrastructure to enable these companies to scale, establish strong UK foundations, and drive forward the transition to a sustainable bioeconomy.
Lord Vallance’s team wrote back to BIA agreeing with our input, saying that the government is committed to delivering the National Vision for Engineering Biology and the aim for the UK to have a broad, rich engineering biology ecosystem that can safely develop and commercialise the many opportunities to come from the technology and the underlying science.
Please contact Senior Policy and Public Affairs Manager Linda Bedenik to find out more about the BIA’s influencing work on engineering biology.
TechBio
BIA hosts workshop to provide input on health data access pricing approach
BIA hosted a workshop for members on pricing approaches for NHS data access. It was ran by the commercial team at the NHS England data for R&D team, who run The NHS Research Secure Data Environment Network. It was an important opportunity to highlight the needs of SMEs to this strategic programme.
After learning about the commercial principles and pricing approach, BIA members had the opportunity to share their feedback, which included that the NHS should:
- Avoid overestimating data value and allow price phasing that can grow with partnerships
- Consolidate resources with a central data team and avoid overly complex financial models
- Build trust and pricing power through reliability, clear communication, and competitive terms to maintain the attractiveness of NHS data.
This is part of an ongoing relationship with the NHSE data for R&D team, who are leading on a network of 11 regional NHS SDEs to support research.
Sudlow Review tells government that BIA should be part of future data advisory group
The Sudlow review on Uniting the UK’s health data was published in November 2024. First commissioned in March 2023, the review makes a comprehensive assessment of the UK’s health data landscape. BIA submitted a response to the review in July 2023.
The review specifically calls for the BIA to feature on the governing board of a new “national data service”. The data service is proposed as a vehicle for accelerating innovation through improved data access. A coordinated joint strategy for health data which oversees the service is also called for. The strategy and service will have key implications for the life sciences sector and could ensure much complexity is reduced. Importantly for us, delivery… “via a strategic partnership with NHS, academic and industry users” is recommended here. This is a big win for BIA, who called for a standardised national data access system in our submission to the review. BIA are continuing to engage with government on the implementation of the Sudlow Review recommendations.
If you are interested in finding out more about the BIA’s work on genomics, data, and AI, including the NHSE Data for R&D Programme please contact Head of Data Tech Policy and Public Affairs, Dr Emma Lawrence.






















