UK companies driving cell and gene therapy: Orchard Therapeutics case study

Orchard Therapeutics is a biotechnology company dedicated to bringing transformative gene therapies to patients with serious and life-threatening orphan diseases. The latest BIA's UK cell and gene therapy report explores the usage of Orchard Therapeutics' hematopoietic stem cell (HSC) gene therapy, as well as the challenges and opportunities of the innovation.
What does the company do?
Orchard Therapeutics is a global gene therapy leader focused on ending the devastation caused by genetic and other severe diseases by discovering, developing and commercialising new treatments that tap into the curative potential of hematopoietic stem cell (HSC) gene therapy. Orchard’s approach harnesses the unique power of a patient’s own genetically modified blood stem cells, also known as hematopoietic stem cells, or HSCs, to potentially treat the underlying cause of a genetic disease permanently with a one-time treatment.
How will it be used?
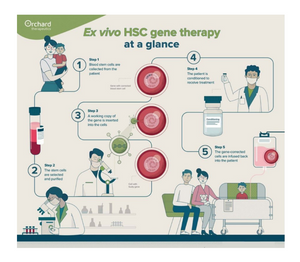
In the HSC gene therapy approach pioneered by Orchard Therapeutics, working copies of the faulty gene causing the disease is inserted and integrated into the genome of the patients’ own HSCs, so that, once engrafted, it is passed on to the cell’s progeny upon each division. This approach offers stable gene correction in HSCs, creating the potential for lasting disease correction.
The company has one approved therapy in Europe and the UK for a rare and fatal pediatric disease and is applying its approach to other severe genetic disorders where it believes its technology is scientifically and clinically differentiated.
What is the impact?
HSCs are responsible for the life-long, sustained production of all blood cells and have three characteristics that make them ideal for personalised gene therapy:
- Self-renewing capacity, to enable a potentially durable response and long-lasting therapeutic effects
- Differentiation into multiple cell types in the blood
- Migration into various tissues and organs, enabling delivery of therapeutic enzymes and proteins to tissues and organs that are often inaccessible to other therapeutic modalities HSC gene therapy has the potential to make a profound difference in the lives of those affected by genetic and other severe diseases. To date across the company’s current and former programs, more than 160 people have been treated in clinical trials with its HSC gene therapies, with more than 12 years of follow-up in the earliest treated patients.
What are the opportunities?
HSCs generate a large variety of differentiated cells in the body, from circulating red or white blood cells to specialized cells lining the gut or those capable of infiltrating the brain, enabling broad distribution of gene-corrected cells and localized and continuous delivery of therapeutic enzymes and proteins at clinically relevant concentrations not achievable by other modalities.
The company has already demonstrated both the safety and clinical efficacy of HSC gene therapy in a rare, fatal disease known as metachromatic leukodystrophy (MLD). In its most severe form, MLD robs a child of their ability to talk, walk and engage with their families and the world around them with the majority of patients passing away within five years of symptom onset of disease symptoms.
The company is exploring its potential to treat other severe conditions, such as mucopolysaccharidosis type I Hurler syndrome, mucopolysaccharidosis type IIIA, also known as Sanfilippo syndrome type A, as well as genetic subsets of frontotemporal dementia and Crohn’s disease.
HSC gene therapy is also well-suited to address severe autoimmune disorders due to the ability of HSCs to differentiate into regulatory T-cells, or Tregs, which are a specialized subset of T cells that can suppress inflammation and be harnessed as a cell therapy with an approach similar to that used to create chimeric antigen receptor T cells, or CAR-Ts. Orchard’s approach aims to combine the demonstrated durability of HSC gene therapy in genetic diseases with the specific suppressive potential of Tregs. Orchard has established a proprietary position covering the concept, therapeutic application and specifics of HSCs antigen-specific Treg therapy.
In addition, Orchard is pursuing the application of HSC as a delivery vehicle of monoclonal antibodies in the patient’s body. This approach has potential advantages over standard administration in terms of efficacy and improved targeting within tissues that are inaccessible by conventional delivery modalities, such as the central nervous system.
1. Mahmood et al. Metachromatic Leukodystrophy: A Case of Triplets with the Late Infantile Variant and a Systematic Review of the Literature. Journal of Child Neurology 2010, DOI: http://doi.org/10.1177/0883073809341669
UK-NoP-2300017 | DOP: Nov 2023
More news and updates
.png)
.png)