mRNA Revolution: eXmoor case study

eXmoor is a leading technical and strategic consultancy specialising in cell and gene therapies (ATMPs) and biopharmaceuticals. In this case study, the BIA's report mRNA Revolution: A new generation of medicine explores eXmoor's biomanufacturing offerings, including its facilities and expertise and its commercialisation lab facilities in Bristol.
What does the company do?
eXmoor is a one-stop cell and gene therapy CDMO (contract development and manufacturing organisation), accelerating the manufacturing journey from research to patients. The company has grown over the last 19 years from supporting clients through consultancy for capital and technical projects and now offers in-house process development and GMP manufacturing. Phase 1 of eXmoor’s new purpose-built 65,000 ft2 facility includes four clean rooms, a fill and finish suite and a range of laboratories for process development, analytical development and quality control.
In August 2023 eXmoor formed a global partnership with Kincell Bio, a US-based CDMO that seamlessly brings together both companies’ capabilities, giving customers the opportunity to access a global development and manufacturing footprint in combination with a boutique customer-focused offering.
How will RNA be used?
The COVID-19 pandemic highlighted the significant benefits of mRNA-based vaccines, helping to accelerate the applications of this technology beyond just infectious diseases to other areas such as enzyme replacement and oncology. This has resulted in increased demand for mRNA manufacturing capacity.
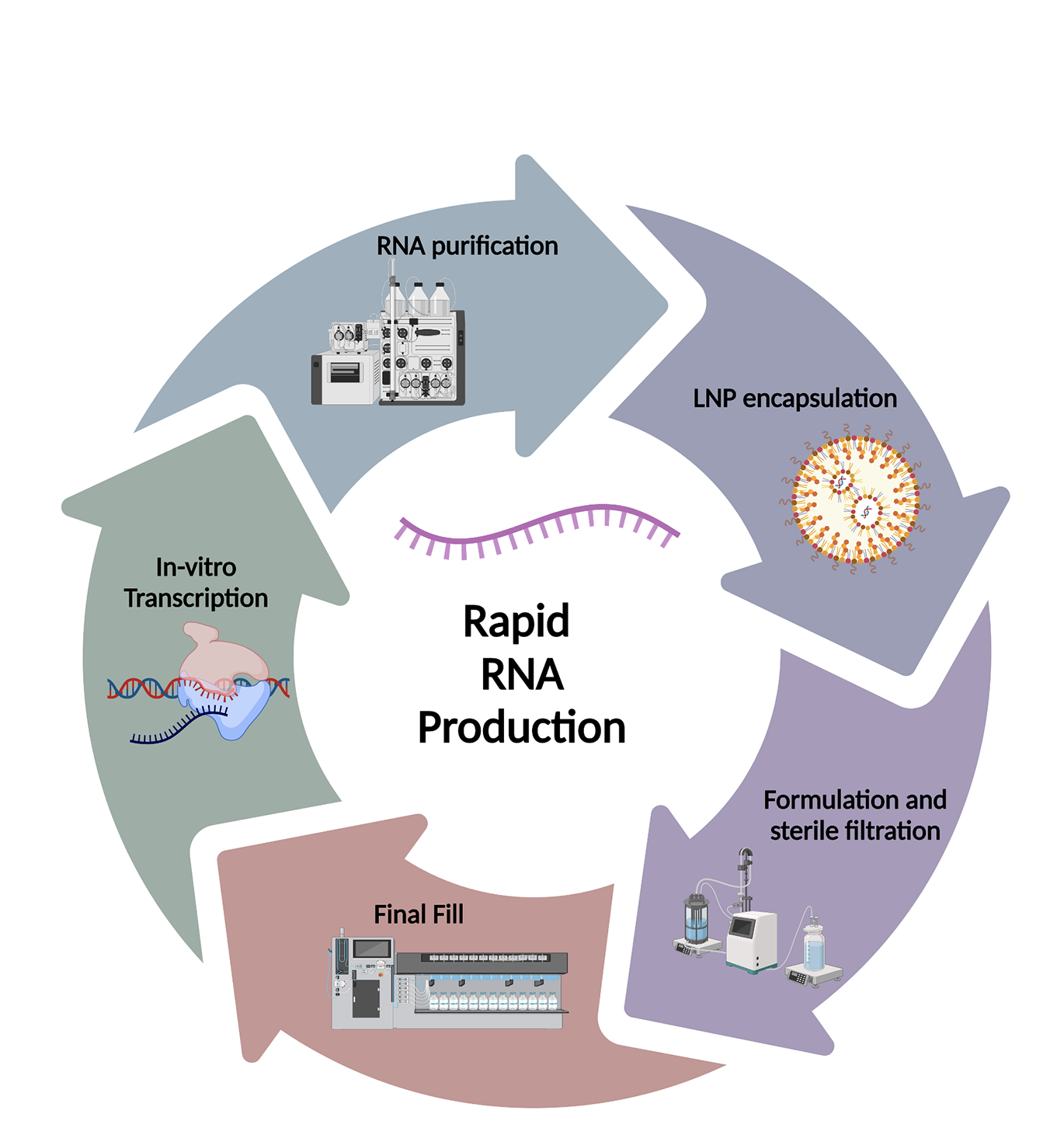
What are your challenges?
mRNA manufacture has unique challenges, and some learning is better transferred from skills developed in cell and gene therapy manufacture rather than classic biologics. eXmoor is uniquely positioned to help with additional capacity and specialist expertise.
Normally, as the scale of a biomanufacturing process increases the price per dose is reduced. But with RNA, driven by the high raw material costs, scale-up results in significant financial risk but only a limited reduction in the price per dose. Vaccine manufacture requires larger batch sizes so process development is likely to focus on efficient use and cost reduction of raw material, as this will have the biggest impact on the price per dose. But not all applications of mRNA need large-scale manufacture and are more sensitive to price per batch than price per dose.
The high raw material cost used to manufacture mRNA significantly impacts the distribution of costs when working at larger IVT volume as shown below.
What does the future look like for eXmoor?
To support the additional mRNA manufacturing requirements, eXmoor is expanding its portfolio to include an end-to-end service for the manufacture of early-stage mRNA products and therapeutics focusing on flexible scale, rapid turn around and responsive material supply.
eXmoor’s approach is designed to meet the needs of customers requiring smaller batch sizes and will target IVT scales of up to 0.5l.
More news and updates
.png)
.png)